LIFE TAKES MUSCLE
Exploring a new focus on muscle-targeted treatments for adults and children living with SMA.
MOVING SMA UNDERSTANDING INTO THE FUTURE
Survival motor neuron (SMN)-targeted treatments have transformed outcomes for people living with SMA, achieving progress beyond what was previously believed possible.1
Lyza, 23
Living with SMA and advocating for progress
Despite these advances, progression and severity of SMA still persist.1,2
Many adults and children living with SMA treated with SMN-targeted treatment still experience muscle wasting and functional deficits.1–3
REAL PATIENTS, REAL STORIES
Listen to a special Life Takes Muscle Podcast episode with Dr. Diana Castro.

People Living with SMA Discuss the Impacts of Progressive Muscle Weakness
In this episode: Host Diana Castro, MD, leads a discussion with three adults living with spinal muscular atrophy (SMA). Listen in as Andrew, Lyza, and Doug share their firsthand experience on how SMA not only impacts their daily lives, but their future goals. They shed light on the nuances of progressive muscle weakness and loss of motor function and the need for more in SMA to help maintain their independence.
TIME IS MUSCLE
For people with SMA, timing matters.
Muscle deficits are most challenging for those treated later in the disease course. Thus, beginning treatment as early as possible is seen as critical.4,5 And while current treatments have dramatically changed expectations for the progression of the disease, adults and children living with SMA can still experience a progressive loss of muscle over time while on SMN-targeted treatments.3,6–8
THE UNMET NEED
In SMA, despite ongoing SMN-targeted treatment, motor function decline can continue to progress.7,8
Change in HFMSE over 7 years with nusinersen7
CHERISH was a phase 3, randomized, double-blind, sham-controlled study to evaluate the efficacy and safety of nusinersen in children with later-onset SMA.9 SHINE was an open-label extension study for patients with SMA who previously participated in investigational studies of nusinersen, such as CHERISH.10
HFMSE=Hammersmith Functional Motor Scale—Expanded; SD=standard deviation; SE=standard error
Change in HFMSE over 5 years with risdiplam8
SUNFISH was a 2-part clinical trial of risdiplam in a broad and heterogeneous patient population with types 2 and 3 SMA (aged 2-25 years).8
aClinical cutoff date: October 2, 2023. bClinical cutoff date: September 6, 2019. Patients in the placebo group received placebo for 12 months followed by risdiplam treatment for 48 months. *±95% CI. Baseline is the last
measurement prior to the first dose of risdiplam or placebo. §Number of patients with valid results=number of patients with an available total score (result) at respective time points. Intent-to-treat patients
HFMSE=Hammersmith Functional Motor Scale—Expanded; SD=standard deviation; SE=standard error
a 2024 survey indicated that even on treatment6:
95% of children and 100% of adults reported muscle weakness persists.
Severity of Muscle Weakness Experienced by Children and Adults Living with SMA6
Mckenna, 16
Living with SMA and advocating for progress
In the same 2024 survey, the 3 most frequently reported burdensome impacts on people living with SMA were6:
Most Burdensome Impacts of SMA6
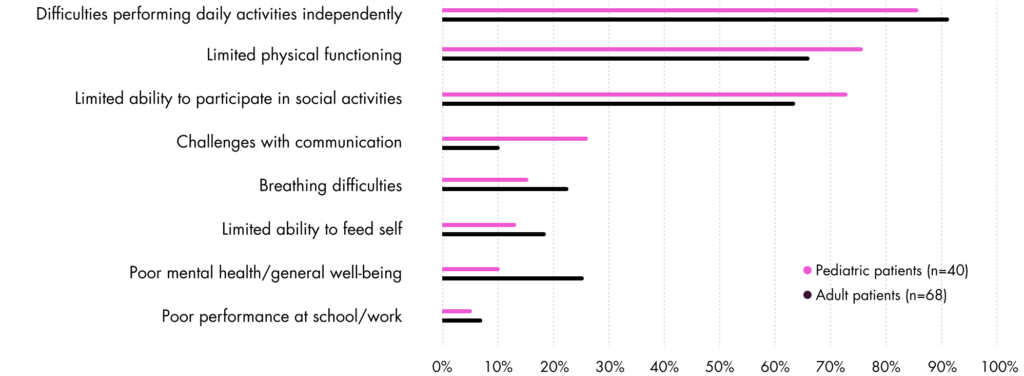
INDEPENDENCE IS ON THE LINE13
Activities of daily living such as dressing, getting out of bed, self-care, self-feeding, picking up and holding objects, walking, and using touchscreen devices are affected by progressive muscle wasting, directly impacting independence.13
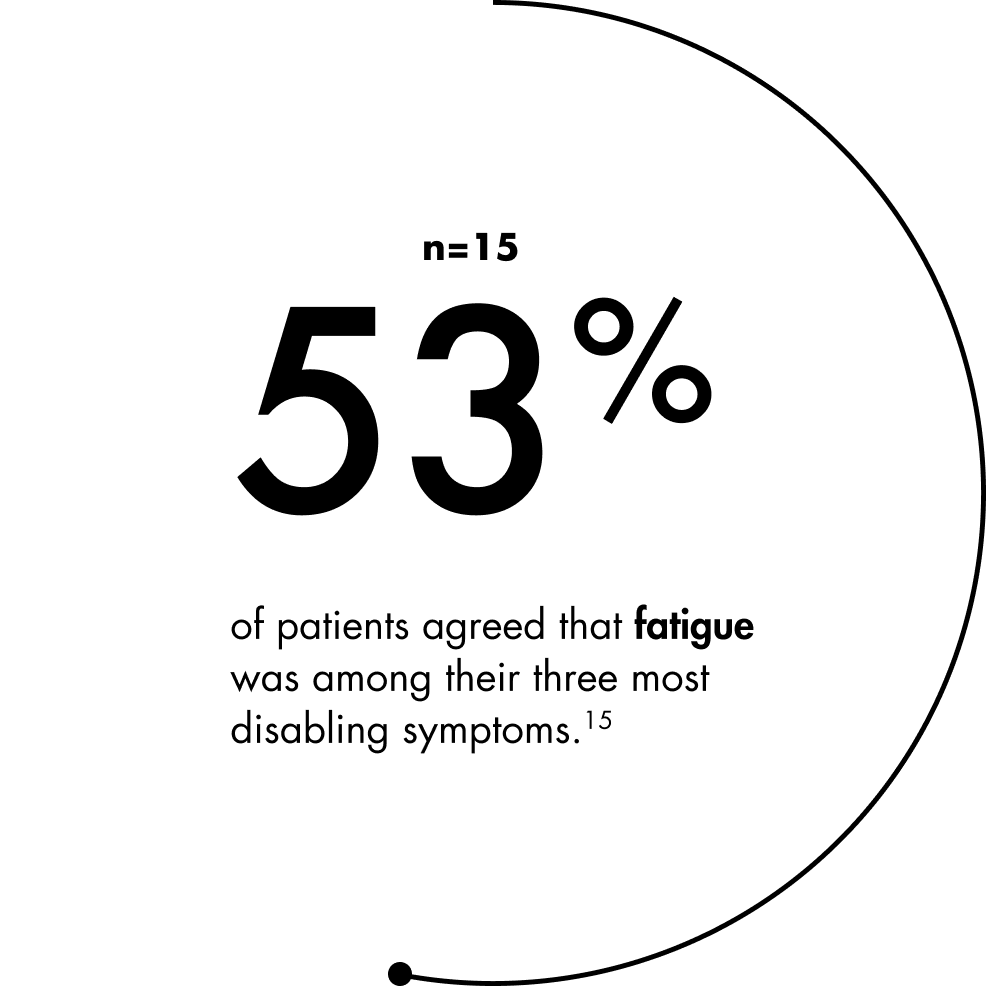
LIVING WITH FATIGUE
Fatigue is seen as one of the most disabling symptoms of SMA.14,15 Studies suggest the dimensions of physical fatigue, general fatigue, and reduced activity are the most severely impacted, but individuals with SMA also report other dimensions of fatigue, such as mental fatigue.14,15
THE SMA COMMUNITY NEEDS MORE
When asked what unmet needs they want new treatments to address, 89% of people living with SMA said muscle strength, 71% said reduced fatigue, and 64% said new motor function.16
Data derived from the 2024 Cure SMA Community Update Survey, an annual online survey (n=302).
DEFINING MEANINGFUL CHANGE IN SMA
Although measures of motor function are of interest, tools like HFMSE and Revised Upper Limb Module for SMA (RULM) might not be sensitive enough to capture the personal and practical consequences of weakened muscles in real life.17
Future outcome measures should assess important features of life with SMA, including the ability to perform daily activities, respiratory function, swallowing, fatigue, and endurance.17
People living with SMA, along with their clinicians and caregivers, emphasize that even small improvements can make a meaningful difference in the day-to-day lives of people with SMA.17
Better physical endurance, reduced stiffness, and the acquisition of new abilities are seen as beneficial for many. For others, the maintenance of abilities alone is an important outcome.17
References
- Antonaci L, Pera MC, Mercuri E. New therapies for spinal muscular atrophy: where we stand and what is next. Eur J Pediatr. 2023;182(7):2935-2942.
- Abati E, Manini A, Comi GP, Corti S. Inhibition of myostatin and related signaling pathways for the treatment of muscle atrophy in motor neuron diseases. Cell Mol Life Sci. 2022;79(7):374.
- Day JW, Howell K, Place A, et al. Advances and limitations for the treatment of spinal muscular atrophy. BMC Pediatr. 2022;22(1):632.
- Gnazzo M, Pisanò G, Baldini V, et al. Myostatin modulation in spinal muscular atrophy: A systematic review of preclinical and clinical evidence. Int J Mol Sci. 2025;26(12).
- Schroth M, Deans J, Arya K, et al. Spinal muscular atrophy update in best practices: recommendations for diagnosis considerations. Neurol Clin Pract. 2024;14(4):e200310.
- Parsons JA, Land N, Maravic MC, et al. Remaining burden of spinal muscular atrophy among treated patients: A survey of patients and caregivers. Ann Clin Transl Neurol. 2025;12(10):2020-2035.
- Finkel RS, Farrar MA, Saito K, et al. Final Safety and Efficacy Data From the SHINE Study in Participants With Infantile-Onset and Later-Onset SMA. Poster presented at: Annual Cure SMA Research and Clinical Care Meeting; June 2024; Austin, TX.
- Servais L, Oskoui M, Day J, et al. SUNFISH Parts 1 and 2: 4-year Efficacy and Safety Data of Risdiplam in Types 2 and 3 Spinal Muscular Atrophy (SMA). Poster presented at: Muscular Dystrophy Association (MDA) Clinical and Scientific Conference; March 2025; Dallas, TX.
- Mercuri E, Darras BT, Chiriboga CA, et al. Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. N Engl J Med. 2018;378(7):625-635.
- A Study for Participants With Spinal Muscular Atrophy (SMA) Who Previously Participated in Nusinersen (ISIS 396443) Investigational Studies (SHINE). ClinicalTrials.gov identifier: NCT02594124. Updated September 27, 2024. Accessed December 10, 2025. https://clinicaltrials.gov/study/NCT02594124
- Ch’ng GS, Koh K, Ahmad-Annuar A, et al. A mixed method study on the impact of living with spinal muscular atrophy in Malaysia from patients’ and caregivers’ perspectives. Orphanet J Rare Dis. 2022;17(1):200.
- Wan HWY, Carey KA, D’Silva A, Kasparian NA, Farrar MA. “Getting ready for the adult world”: how adults with spinal muscular atrophy perceive and experience healthcare, transition and well-being. Orphanet J Rare Dis. 2019;14(1):74.
- Duong T, Braid J, Staunton H, et al. Understanding the relationship between the 32-item motor function measure and daily activities from an individual with spinal muscular atrophy and their caregivers’ perspective: a two-part study. BMC Neurol. 2021;21(1):143.
- Rodriguez-Torres RS, Uher D, Gay EL, et al. Measuring fatigue and fatigability in spinal muscular atrophy (SMA): challenges and opportunities. J Clin Med. 2023;12(10).
- Binz C, Schreiber-Katz O, Kumpe M, et al. An observational cohort study on impact, dimensions and outcome of perceived fatigue in adult 5q-spinal muscular atrophy patients receiving nusinersen treatment. J Neurol. 2021;268(3):950-962.
- Welsh EF. Belter L, Whitmire SM, Curry M, Schroth M. Unmet Needs Among Adults Living with Spinal Muscular Atrophy in the United States. Poster presented at: Muscular Dystrophy Association (MDA) Clinical and Scientific Conference; March 2025; Dallas, TX.
- McGraw S, Qian Y, Henne J, Jarecki J, Hobby K, Yeh W-S. A qualitative study of perceptions of meaningful change in spinal muscular atrophy. BMC Neurol. 2017;17(1):68.
- Mercuri E, Finkel RS, Muntoni F, et al. Diagnosis and management of spinal muscular atrophy: Part 1: Recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul Disord. 2018;28(2):103-115.
- Boyd PJ, Gillingwater TH. Axonal and Neuromuscular Junction Pathology in Spinal Muscular Artophy. In: Sumner CJ, Paushkin S, Ko CP, eds. Spinal Muscular Atrophy: Disease Mechanisms and Therapy. Elsevier; 2017:133-151.
- Le Verche V, Sunshine SS, Hammers D. Skeletal Muscle in Spinal Muscular Atrophy As an Opportunity for Therapeutic Intervention. In: Sumner CJ, Paushkin S, Ko CP, eds. Spinal Muscular Atrophy: Disease Mechanisms and Therapy. Elsevier; 2017:341-356.
- Deguise MO, Patitucci TN, Ebert AD, Lorson CL, Kothary R. Contributions of Different Cell Types to Spinal Muscular Atrophy Pathogenesis. In: Sumner CJ, Paushkin S, Ko CP, eds. Spinal Muscular Atrophy: Disease Mechanisms and Therapy. Elsevier; 2017:167-181.
- Jha NN, Kim J-K, Her Y-R, Monani UR. Muscle: an independent contributor to the neuromuscular spinal muscular atrophy disease phenotype. JCI Insight. 2023;8(18).
- Wirth B, Mendoza-Ferreira N, Torres-Benito L. Spinal Muscular Artophy Disease Modifiers. In: Sumner CJ, Paushkin S, Ko CP, eds. Spinal Muscular Atrophy: Disease Mechanisms and Therapy. Elsevier; 2017:191-210.
- Durmus H, Yilmaz R, Gulsen-Parman Y, et al. Muscle magnetic resonance imaging in spinal muscular atrophy type 3: Selective and progressive involvement. Muscle Nerve. 2017;55(5):651-656.
- Deymeer F. Natural history of SMA IIIb. Neurology. August 26, 2008.
- Buchthal F, Olsen PZ. Electromyography and muscle biopsy in infantile spinal muscular atrophy. Brain. 1970;93(1):15-30.
- Perez-Garcia MJ, Kong L, Sumner CJ, Tizzano EF. Developmental Aspects and Pathological Findings in Spinal Muscular Atrophy. In: Sumner CJ, Paushkin S, Ko CP, eds. Spinal Muscular Atrophy: Disease Mechanisms and Therapy. Elsevier; 2017:21-42.
- McCarthy JJ, Esser KA. Anabolic and catabolic pathways regulating skeletal muscle mass. Curr Opin Clin Nutr Metab Care. 2010;13(3):230-235.
- Lee S-J. Targeting the myostatin signaling pathway to treat muscle loss and metabolic dysfunction. J Clin Invest. 2021;131(9).
- Wagner KR. The elusive promise of myostatin inhibition for muscular dystrophy. Curr Opin Neurol. 2020;33(5):621-628.
- Lee S-J, Bhasin S, Klickstein L, Krishnan V, Rooks D. Challenges and future prospects of targeting myostatin/activin A signaling to treat diseases of muscle loss and metabolic dysfunction. J Gerontol A Biol Sci Med Sci. 2023;78(Suppl 1):32-37.